Is the HSE "Phishing" parents for Informed Consent?
By Martin Healy,
Regret.ie, 08/9/2015
This week first-year parents received HPV vaccine information booklets through the schools to enable them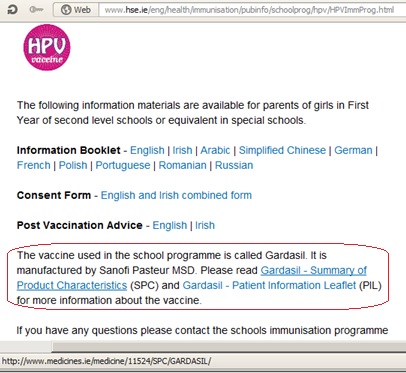 to give ‘informed consent’. Parents not fully convinced of the safety and effectiveness of this vaccine (‘Gardasil’) were invited to visit the HSE website for more info. The HPV School Immunisation section of the website has the following “information for parents”:
to give ‘informed consent’. Parents not fully convinced of the safety and effectiveness of this vaccine (‘Gardasil’) were invited to visit the HSE website for more info. The HPV School Immunisation section of the website has the following “information for parents”:
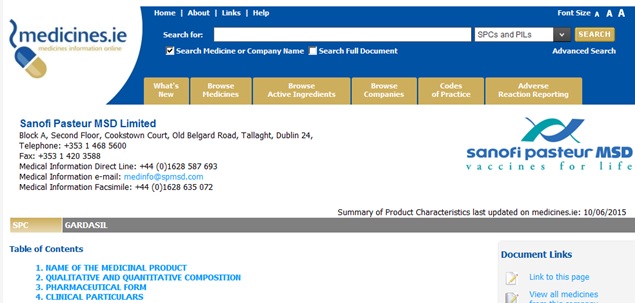
"Gardasil. It is manufactured by Sanofi Pasteur MSD. Please read Gardasil - Summary of Product Characteristics (SPC) and Gardasil - Patient Information Leaflet (PIL) for more information about the vaccine".
The ‘SPC’ link opens a wiki style webpage with a ‘Sanofi Pasteur MSD’ header and official looking logo (see screenshot at bottom of page). At first glance it appears as though you have been directed to the website of the vaccine manufacturer. Similarly for the ‘PIL’ link. These ‘Sanofi Pasteur MSD’ style webpages are in fact located on a “medicines.ie” site which is registered to an Irish lobby group called the IPHA.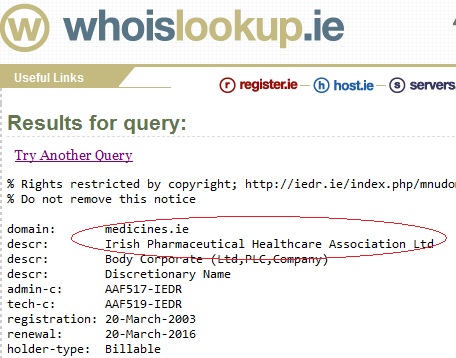
‘Phishing’?
In the IT security world this might be known as ‘Phishing’ – you get directed to an authentic looking payment/banking site (for example), where you enter your login details and account information. However, you are unknowingly on a fake website cleverly designed to imitate the original and fool you into believing that what you see is the real thing.
Who are the IPHA?
The Irish Times reported recently on IPHA lobbying activity when the group highlighted the effects of a new industry pricing agreement aimed at reducing the national drugs bill. The IPHA public affairs manager complained that since the State started paying less for medicines, jobs had been lost and there were “fewer sales reps on the roads” in Ireland. The IPHA group, who only represent the interests of the pharma industry, have now been delegated the responsibility of providing to parents the key information on the safety and effectiveness of the vaccine.
Information contradiction..
However, the Gardasil information on the IPHA site that is being represented as the original manufacturer "Summary of Product Characteristics (SPC)" and "Patient Information Leaflet (PIL)" is not that which it claims to be. Statements such as “Gardasil can be administered according to a 2-dose schedule” (for ages 9- 13) simply do not tally with the actual regulated manufacturer’s documents located on the Merck website.
Merck’s Patient Product Information and Prescribing Information state unequivocally that the schedule always requires three doses, not two. Note that the vaccine maker is MSD (Merck), not “Sanofi Pasteur MSD”* as stated on the HSE site.
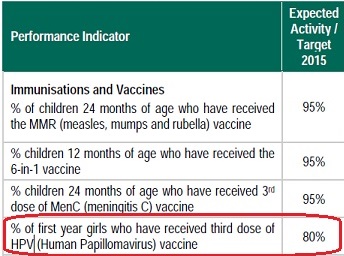
Conflicting targets..
In other countries (such as the U.S), Irish children would be classed as “not fully vaccinated” for HPV having received the first and second dose, but not the third. Even the HSE’s own National Service Plan for 2015 is based on a 3-dose performance target of 80% coverage for: “% of first year girls who have received the third dose of HPV vaccine”. Despite this ‘target’, only 2 doses were given to first year shoolgirls during the 2014/15 schoolyear, and according the the National Immunisation Office, only 2 doses will also be given for the new set of first years for 2015/16.
1 + 1 = 3? 
Can 1 plus 1 ever equal 3? The officials in the National Immunisation Office apparently think so (based on the info they see on the IPHA site?). But the only way that this conundrum could possibly hold true is if 2 doses of the vaccine are equally as ineffective as 3 doses. This is the case when the assumed “protection” offered by 3 doses wears off before the minimum 15-year threshold required for any kind of supposed cancer prevention. The aforementioned HSE authored info booklet gives us a clue: “We will let you know if a booster is needed in the future”.
*The entity "Sanofi Pasteur MSD" is a partnership which only markets the vaccine.